Tim Durham
Florida Gulf Coast University
John Doucet
Nicholls State University
Lori Unruh Snyder
Purdue University
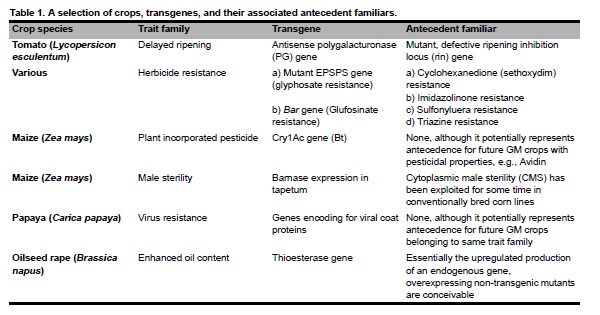
The precautionary principle places an impractical onus on science to demonstrate the absolute safety of genetically-modified (GM) crops. Conversely, traditionally bred articles receive little, if any, regulatory attention. Procedurally, GM certainly has the potential to create end products with deleterious (and thus regulatory actionable) characteristics. However, such risk is ultimately embodied in the end product and not the methodology, per se. Our proposal emphasizes a trait-based, end product model over the method-centric model. Using a de minimus framework, we propose a pragmatic, science-based rubric to assess GM crops. De minimus is designed to minimize regulatory bottlenecks for articles exhibiting nominal risk commensurate with antecedence, while reserving the amenities of precaution for those with an evidently higher risk index. Although GM may pose unique regulatory challenges, it is important that the regulation of risk not turn into the risk of regulation.
Key words: Antecedence, biotechnology, cisgenic, de minimus, genetically modified, precautionary principle, regulation, transgenic.


